Neuroethology of Communication Lab

VOIMA project (ERC Starting Grant, 2021-2026)
Voice and speech perception across mammals: a comparative study of humans, dogs and pigs
Background
Vocalizations of any mammal carry prominent cues about the inner states and identity of the vocalizer. Voice is also a prevalent channel for humans’ recently emerged communication system, speech. Recent evidence suggests that certain human auditory brain specializations and mechanisms, relevant for voice and speech perception, reflect abrupt shifts in human capacities compared to other primates.
Main questions
Do these brain specializations for voice and speech perception reflect human-specific predispositions and are thus human-unique, or are they the consequence of rapid evolutionary adaptations or developmental accommodations of the ancient voice perception system to recent demands imposed by the presence of speech? I hypothesize that in general voice perception mechanisms are conserved across mammals, and provide a neuronal niche in which specializations for human voice and speech perception may arise also in non-humans.
The comparative neuroscientific approach
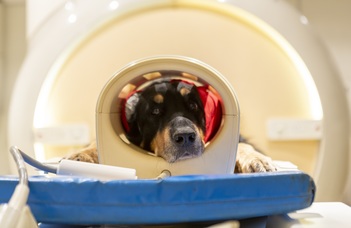
The case of companion animals provides an unparalleled model system to study the possible evolutionary and experiential effects of the presence of speech on the mammalian voice perception system. Dogs and pigs are phylogenetically distant, highly vocal species that live, when kept as companions, with humans. VOIMA combines ethology and brain imaging (EEG/fMRI/HD-DOT) to compare voice and speech processing in humans, dogs and pigs: WP1 seeks evidence for selective processing of conspecific voices, human voice, and speech. WP2 explores the mechanisms and specific sensitivities for inner state coding, voice identity recognition and vocalizer normalization, from con- and heterospecific voice. WP3 tests how sensitivities to human voice and speech emerge across dog breed types, in neonate dogs, pigs, wolves and wild boars, and in input-manipulated developing dogs. Revealing how adaptation to the human social niche shapes domestic mammals' voice perception, this project will provide new insights on how speech shaped human voice perception.
Lendület-project (MTA-ELTE 'Lendület' Neuroethology of Communication Research Group, 2017-2022)
Comparative mammalian brain imaging: a neuroethological approach to the emergence of lexical representation
Background
Lexical items (words) are the basic building blocks of human languages, but previous research hardly ever found items with lexical features in nonhuman vocal communicative systems, even though several species are capable of learning and discriminating arbitrary sound sequences, associating vocalizations with specific meanings, or producing human-like lexical items after extensive training. Recently, we presented the first fMRI study on how dog brain processes human words, demonstrating that dogs, similarly to humans, are capable of storing lexical representations (i.e. arbitrary associations of sound sequences to meanings) independently of paralinguistic features, such as intonation (Andics et al. 2016, Science). This research suggests that human linguistic capacity cannot be understood in isolation. A broad comparative perspective to what brain mechanisms various species have in common with humans for vocal social processing is essential for a better understanding of the contributions of biological and cultural evolution to the emergence of linguistic competence.
Main questions
How and under what ontogenetic conditions do lexical representations emerge in nonhuman brains? How do mammals acquire and consolidate new meanings, and how do they fit them in their pre-existing lexico-semantic network? What are the organizing principles of learnt, vocally encoded meanings, and how do they differ across conspecific and heterospecific vocalizations, and across semantic domains (Binder 2009)? Do mammals share the representations of basic biological meanings (Ehret 2010) in vocal utterances, and can this hard-wired semantics be overruled by learnt sound-to-meaning associations? Are dogs in a better position than other mammals that had not been under a similar selective pressure to fit into the human social environment, to learn the meanings of human words?
The comparative approach
We hypothesize that the shared capacity of dogs and humans to store lexical representations is based on an ancient capacity present in the last common ancestor of the two species. We therefore expect that certain lexical processing capacities, which are shared among dogs and humans, are highly similar to those in other mammals. The domestic pig is an evolutionarily distant relative of both dogs and humans, with a domestication history that is considerably shorter than that of dogs, and that followed a very different trajectory. Nevertheless, if socialized by humans, pigs are also receptive to interspecific social cues, and can follow ostensive-referential communicative cues such as human pointing or gaze direction. When kept as pets, dogs and pigs occupy a similar ecological niche of living with humans. Extending comparative neuroscientific studies on vocal social functions to pigs could therefore shed light on the biological and cultural determinants of lexical processing, revealing how similar or variable neural representations can get in species with a highly different phylogenetic history, once the environment is stabilized, and potentially highly controlled.
Contact
Attila Andics (group leader), attila.andics@ttk.elte.hu
Video abstracts
https://www.youtube.com/watch?v=4-g72gaO7fo&t=7s
https://www.youtube.com/watch?v=uwWraDFDeR4
https://www.youtube.com/watch?v=-jt-PJDb_gA&t=1s
https://www.youtube.com/watch?
https://www.youtube.com/watch?
https://www.youtube.com/watch?
https://www.youtube.com/watch?
https://www.youtube.com/watch?
https://www.youtube.com/watch?
https://www.youtube.com/watch?
https://www.youtube.com/watch?
https://www.youtube.com/watch?
https://www.youtube.com/watch?
https://www.youtube.com/watch?
https://www.youtube.com/watch?
https://www.youtube.com/watch?
https://www.youtube.com/watch?
https://www.youtube.com/watch?
Publications
Gergely, A., Gábor, A., Gácsi, M. et al. (2023) Dog brains are sensitive to infant- and dog-directed prosody. Commun Biol 6, 859 [pdf][link][VA]
Kőszegi, H., Fugazza, C., Magyari, L. et al. (2023) Investigating responses to object-labels in the domestic dog (Canis familiaris). Sci Rep 13, 3150. https://doi.org/10.1038/
Lehoczki, F., Andics, A., Kershenbaum, A. et al. (2023) Genetic distance from wolves affects family dogs’ reactions towards howls. Commun Biol 6, 129 . https://doi.org/10.1038/s42003-023-04450-9 [pdf][link][VA]
Pérez Fraga, P., Morvai, B., Gerencsér, L. et al. (2023) Out-of-reach rewards elicit human-oriented referential communicative behaviours in family dogs but not in family pigs. Sci Rep 13, 811. https://doi.org/10.1038/s41598-022-26503-5 [pdf][link][VA]
Bálint A, Szabó Á, Andics A, Gácsi M. (2023) Dog and human neural sensitivity to voicelikeness: A comparative fMRI study. NeuroImage 265:119791. doi: 10.1016/j.neuroimage.2022.
Bálint, A., Eleőd, H., Magyari, L. et al. (2022) Differences in dogs’ event-related potentials in response to human and dog vocal stimuli; a non-invasive study. Royal Society open science, 9(4), 211769 [pdf][link]
Gábor A, Kaszás N, Faragó T, Pérez Fraga P, Lovas M, Andics A (2022) The acoustic bases of human voice identity processing in dogs. Animal Cognition https://doi.org/10.1007/s10071-022-01601-z [pdf][link][VA]
Cuaya LV, Hernández-Pérez R, Boros M, Deme A, Andics A (2022) Speech naturalness detection and language representation in the dog brain. NeuroImage https://doi.org/10.1016/j.neuroimage.2021.118811 [pdf][link][VA]
Boros M, Magyari L, Török D, Bozsik A, Deme A, Andics A (2021) Neural processes underlying statistical learning for speech segmentation in dogs. Current Biology 31, 1–10, https://doi.org/10.1016/j.cub.2021.10.017 [pdf][link][VA]
Gábor A, Andics A, Miklósi Á, Czeibert K, Carreiro C, Gácsi M (2021) Social relationship-dependent neural response to speech in dogs. NeuroImage, 243, 118480. https://doi.org/10.1016/j.neuroimage.2021.118480 [pdf][link][VA]
Fugazza C, Andics A, Magyari L, Dror S, Zempléni A, Miklósi Á (2021) Rapid learning of object names in dogs. Scientific Reports, 11, 2222. doi: 10.1038/s41598-021-81699-2 [pdf][link]
Gunde E, Czeibert K, Gábor A, Szabó D, Kis A, Arany-Tóth A, Andics A, Gácsi M, Kubinyi E (2020) Longitudinal volumetric assessment of ventricular enlargement in pet dogs trained for functional magnetic resonance imaging (fMRI) studies. Veterinary Sciences, 7: 127. doi: 10.3390/vetsci7030127 [pdf][link]
Magyari L, Huszár Zs, Turzó A, Andics A (2020) Event-related potentials reveal limited readiness to access phonetic details during word processing in dogs. Royal Society Open Science, 7: 200851. doi: 10.6084/m9.figshare.c.5221448 [pdf][link][VA]
Pérez Fraga P, Gerencsér L, Andics A (2020) Human proximity seeking in family pigs and dogs. Scientific Reports 10, 20883. https://doi.org/10.1038/s41598-020-77643-5 [link][VA]
Bunford N, Hernández-Perez R, Farkas E, Cuaya L, Szabó D, Szabó Á, Gácsi M, Miklósi Á, Andics A (2020) Comparative Brain Imaging Reveals Analogous And Divergent Patterns Of Species- And Face-Sensitivity In Humans And Dogs. The Journal of Neuroscience, JN-RM-2800-19; DOI: 10.1523/JNEUROSCI.2800-19.2020 [pdf][link][VA]
Gábor A, Gácsi M, Szabó D, Miklósi Á, Kubinyi E, Andics A (2020) Multilevel fMRI adaptation for spoken word processing in the awake dog brain. Scientific Reports 10, 11968 https://doi.org/10.1038/s41598-020-68821-6 [pdf][SM][link][VA]
Pérez Fraga P, Gerencsér L, Lovas M, Újváry D, Andics A (2020) Who turns to the human? Comparing pigs’ and dogs’ behaviour in the unsolvable task paradigm. Animal Cognition. doi: 10.1007/s10071-020-01410-2 [pdf][link][VA]
Boros M, Gábor A, Szabó D, Bozsik A, Gácsi M, Szalay F, Faragó T, Andics A (2020) Repetition enhancement to voice identities in the dog brain Scientific Reports 10, 3989 https://doi.org/10.1038/s41598-020-60395-7 [pdf][link][VA]
Bálint A, Andics A, Gácsi M, Gábor A, Czeibert K, Luce CM, Miklósi Á, Kröger RHH (2020) Dogs can sense weak thermal radiation. Scientific Reports 10, 3736 https://doi.org/10.1038/s41598-020-60439-y [pdf][link][VA]
Szabó D, Gábor A, Gácsi M, Faragó T, Kubinyi E, Miklósi Á and Andics A (2020) On the Face of It: No Differential Sensitivity to Internal Facial Features in the Dog Brain. Frontiers Behavioral Neuroscience. 14:25. doi: 10.3389/fnbeh.2020.00025 [pdf][link][VA]
Andics A, Faragó T (2019) Voice perception across species. In: S. Frühholz, P. Belin (Eds.), Oxford Handbook of Voice Perception. Oxford, UK: Oxford University Press (pp. 363-392). doi: 10.1093/oxfordhb/9780198743187.013.16 [link]
Czeibert K, Andics A, Petneházy Ö, Kubinyi E (2019) A detailed canine brain label map for neuroimaging analysis. Biologia Futura, 70: 112-120, doi: 10.1556/019.70.2019.14 [pdf][link]
Gábor A, Kaszás N, Miklósi Á, Faragó T, Andics A (2019) Interspecific voice discrimination in dogs. Biologia Futura, 70: 121–127. doi: 10.1556/019.70.2019.15 [pdf][link]
Gerencsér L, Pérez Fraga P, Lovas M, Újváry D, Andics A (2019) Comparing interspecific socio-communicative skills of socialized juvenile dogs and miniature pigs. Animal Cognition 22: 917, doi:10.1007/s10071-019-01284-z [pdf][link][VA]
Szabó D, Czeibert K, Kettinger Á, Gácsi M, Andics A, Miklósi Á, Kubinyi E (2019) Resting-state fMRI data of awake dogs (Canis familiaris) via group-level independent component analysis reveal multiple, spatially distributed resting-state networks. Scientific Reports 9, 15270. doi: 10.1038/s41598-019-51752-2 [pdf][link]
Andics A, Miklósi Á (2018) Neural processes of vocal social perception: Dog-human comparative fMRI studies, Neuroscience & Biobehavioral Reviews 85: 54-64. [pdf][link]
Gerencsér L, Bunford N, Moesta A, Miklósi Á (2018) Development and validation of the Canine Reward Responsiveness Scale –Examining individual differences in reward responsiveness of the domestic dog, Scientific Reports, 8, 4421. doi:10.1038/s41598-018-22605-1 [pdf][link]
Bunford N, Andics A, Kis A, Miklósi Á, Gácsi M (2017) Canis familiaris As a Model for Non-Invasive Comparative Neuroscience, Trends in Neurosciences, 40(7): 438-452. [pdf][link]
Andics A, Gábor A, Gácsi M, Faragó T, Szabó D, Miklósi Á (2016) Neural mechanisms for lexical processing in dogs. Science, 353: 1030-1032. doi: 10.1126/science.aaf3777 [pdf][SM][link][VA]
Andics A, Gácsi M, Faragó T, Kis A, Miklósi Á (2014) Voice-sensitive regions in the dog and human brain are revealed by comparative fMRI. Current Biology, 24: 574-578. [pdf][SM] [link][VA]

